The substituted xanthines are a good example of an isomer found in food and drugs. 3 Chemical and Physical Properties Expand this section.
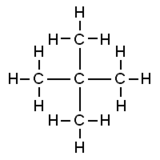
Which is an isomer of 2 2 dimethylpropane.
2 2 dimethylpropane. Neopentane also called 22-dimethylpropane is a double-branched-chain alkane with five carbon atoms. Neopentane is a flammable gas at room temperature and pressure which can condense into a highly volatile liquid on a cold day in an ice bath or when compressed to a higher pressure. Neopentane is the simplest alkane with a quaternary carbon and has achiral tetrahedral symmetry.
1 Structures Expand this section. 2 Names and Identifiers Expand this section. 3 Chemical and Physical Properties Expand this section.
4 Spectral Information Expand this section. C 5 H 12. 1 Structures Expand this section.
2 Names and Identifiers Expand this section. 3 Chemical and Physical Properties Expand this. Isomer 3 is 22-dimethylpropane a branched chain with the central carbon atom joined onto four other carbon atoms.
Note that for all the isomers each carbon atom has four bonds valency 4 and each hydrogen atom has one bond valency 1. Valency is the combining power of an atom. You cannot change the number of bonds.
The reaction is Sn2 and even though 1-chloro-22-dimethylpropane is a primary chloride it is more sterically hindered than 2-chloropropane which is secondary. Cl HC C Na H Na Cl Cl 1-chloro-22-dimethylpropane main organic product 9 This is purely a solvent effect. The greater the percentage of water in this solvent mixture the more polar the solvent.
The more polar the solvent the. Answer 1 of 8. Pentane shows structural isomerism of three types.
If you have the problem with calculating isomers of alkane just try to write the structure of the compound that is the easiest way to find out the isomerism of alkane. Likewise this you can calculate the structural isomerism of. 22-Dimethylpropane C5H12 49 _____ Disilane Si2H6 26 _____ Ethane C2H6 118 354 Ethyl Chloride C2H5Cl 55 _____ Ethylene Ethene C2H4 127 601 Ethylene Oxide C2H4O 81 605 Fluoroform See also Freon 23 CHF2 53 _____ Fluorine F2 94 1179.
Forane 134a Freon134a C2H2F4 35 _____ Freon-11 Trichloro-fluoromethane CCl3F 26 279 Freon-12 Dichloro-difluoromethane CCl2F2 29 325 Freon. 22-dimethylpropane So it is probably time for some practice problems. Name the following compound or draw its structure.
A answer b 2-methyl-4-ethylheptane answer. Thermophysical Properties of Fluid Systems. Accurate thermophysical properties are available for several fluids.
These data include the following. The reaction is an example of a nucleophilic addition b free radical addition c electrophilic addition d electrophilic substitution. Halogen acids react with alcohols to form alkyl halides.
The reaction follows a nucleophilic substitution mechanism. What will be the product of the. Pentane 2-methylbutane and 22-dimethylpropane are structural isomers of each other.
Importance of Isomerism. Isomers are especially important in nutrition and medicine because enzymes tend to work on one isomer over another. The substituted xanthines are a good example of an isomer found in food and drugs.
Theobromine caffeine and theophylline are isomers differing in the. Fuel Gases C Fitting Standard cylinder valve outlet connection for pressures up to 200 psig 1380 kPa875-14UNF-2A-LH-EXT. Typically used for CGA V-1 Connection Number 024 025 034 035.
A increases its density and thus increases its melting point. B decreases its density and thus increases its melting point. C increases its density and thus decreases its melting point.
ChemIDPlus Water Solubility Practically insoluble 38 mgL 25C Insoluble Insoluble ACGIH 2001 Log K ow 339 272 311 ChemIDPlus Vapor Pressure 514 mm Hg 25C 689 mm Hg 25C 1290 mm Hg 25C ChemIDPlus Vapor Density air 1 248 — — ChemIDPlus Density 06262 20C 06197 20C 0591 20C ACGIH 2001 Melting Point -1297C -1599C -166C. 2825 K V 2019-20. 376 CHEMISTRY to V.
If you go on constructing structures for higher alkanes you will be getting still larger number of isomers. C6H14 has got five isomers and C7H16 has nine. As many as 75 isomers are possible for C10H22.
In structures II IV and V you observed that CH3 group is attached to carbon atom numbered as 2. N-pentane 2-methylbutane 22-dimethylpropane 36 C 28 C 9 C As branching increases the strength of the van der Waals interactions between molecules decreases resulting in the lowering of boiling points. Occurrence Natural gas - principally methane CH 4 Petroleum oil - mixture up to ca.
N 40 Separation of crude oil is achieved by fractional. The molecular mass of 2 2-Dimethylpropane. 72 g mol-1 The molecular mass of 2-Methylbutane.
72 g mol-1 2-Methylpropane has the lowest molecular mass among all of the given compounds. Thus 2-Methylpropane has the lowest boiling point among the given options. Among the following the maximum covalent character is shown by the compound a MgCl 2 b FeCl.
CBSE Class 11 Chemistry Organic Chemistry Some Basic Principles and Techniques MCQs with answers available in Pdf for free download. The MCQ Questions for Class 11 Chemistry with answers have been prepared as per the latest syllabus NCERT books and examination pattern suggested in Standard 11 by CBSE NCERT and KVS. Multiple Choice Questions are an important part of exams for.
For example 22-dimethylpropane neopentane has a low boiling point than pentane. In shape neopentane is more spherical than that of pentane. Hence it occupies less surface area than the more cylindrical pentane molecule.
As a result the van der Waals forces are smaller in neopentane and the boiling point is low. Generally the boiling points of isomeric alkanes depend on their shapes. 2-methylbutane 22-dimethylpropane There are three isomers of pentane C5H12 Do not draw false isomers which are just twisted versions of the original molecule.
Twisting the molecule into a different shape does not make a different isomer. Isomers are only formed if a bond would have to be broken and reassembled into the different structure C. Which is an isomer of 2 2 dimethylpropane.
What happened in Act 2 Scene 2 Macbeth. How many variables are there in the factorial research design of 2 3 2. What is the size of a 2 1 2 car garage.
What is the molecular formula for alkane 2 2 4 Trimethylpentane. Is 2 chloro 2 Methylpropane sn1 or sn2. What is the difference between a 1 1 2 story and 2.
Cannot tell without more information. How many methyl peaks would you expect to observe in the 1H NMR spectrum of cis-14-dimethylcyclohexane. An unknown compound A has the molecular formula C4H8O2.
Based on the. 7 The complete combustion of 22-dimethylpropane C 4 H 10 in oxygen. 2 C 4 H 10 13 O 2 8 CO 2 10 H 2 O 8 The reaction of AlBr 3 with MgOH 2 2 AlBr 3 3 MgOH 2 2 AlOH 3 3 MgBr 2 9 The decomposition of hydrogen peroxide to form water and oxygen.
2 H 2 O 2 2 H 2 O O 2 10 The reaction of ammonia with sulfuric acid. 22-dimethylpropane 2-methylbutane position isomers. Compounds with the same molecular formula but different structures due to different positions of the same functional group on the same carbon skeleton C C H H Br H C H H H H C C H H H Br C H H H H 1-bromopropane 2-bromopropane C O C H H H H H H C O H H H C H H H ethanol.
An ether C C C C C C H H.