The 2 indicates that the OH group is attached to the second carbon atom. This structure is also available as a 2d Mol file or as a computed 3d SD file The 3d structure may be viewed using Java or Javascript.
Benzoic acid solid and 1-Propanol liquid Ester C.
1 propanol structure. Structure properties spectra suppliers and links for. 1-Propanol Propan-1-ol 71-23-8 71-31-8 109-78-4 927-74-2 36294-23-2. Jump to main content Jump to site nav.
Membership. Journals books. 1-Propanol is a primary alcohol with the formula CH 3 CH 2 CH 2 OH and sometimes represented as PrOH or n-PrOHIt is a colorless liquid and an isomer of 2-propanolIt is formed naturally in small amounts during many fermentation processes and used as a solvent in the pharmaceutical industry mainly for resins and cellulose esters and sometimes as a disinfecting agent.
Propan-1-ol is the parent member of the class of propan-1-ols that is propane in which a hydrogen of one of the methyl groups is replaced by a hydroxy group. It has a role as a protic solvent and a metabolite. It is a short-chain primary fatty alcohol and a member of propan-1-ols.
2-Amino-2-methyl-1-propanol C4H11NO CID 11807 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazardstoxicity information supplier lists and more. Public health information CDC Research information NIH SARS-CoV-2 data NCBI Prevention and treatment information HHS Español. Intermolecular forces in 1 propanol.
Propanol is one of the most common types of alcohol. Propanol has the formula CH 3 CH 2 CH 2 OH. Propan-1-ol n-propyl alcohol 1-propyl alcohol or n-propanol are all names for this colourless oil.
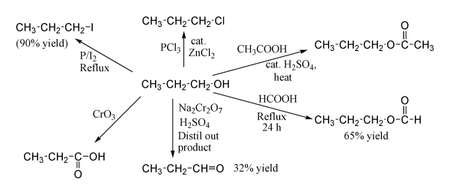
IUPAC Name propan-1-ol. Propionaldehyde is catalytically hydrogenated to produce 1-propanol. Propionaldehyde is made by hydroformylation ethylene.
Alcohol - alcohol - Physical properties of alcohols. Most of the common alcohols are colourless liquids at room temperature. Methyl alcohol ethyl alcohol and isopropyl alcohol are free-flowing liquids with fruity odours.
The higher alcoholsthose containing 4 to 10 carbon atomsare somewhat viscous or oily and they have heavier fruity odours. Structure and properties Index of refraction n 25. 1361 Dielectric constant ε r.
243 ε 0 at 20 C Bond strength. 5810 7 cgs units volume Surface tension. 2239 dyncm at 25 C Thermal conductivity.
01660 W m 1 K 1 saturated liquid at 300 K Viscosity. At 50 C 4656 mPas. At 40 C 3530 mPas.
257E-009 Octanolair Koa model. 492E-009 Fraction sorbed to airborne particulates phi. 927E-008 Mackay model.
205E-007 Octanolair Koa model. 394E-007 Atmospheric Oxidation 25 deg C AopWin v192. OVERALL OH Rate Constant 130448 E-12 cm3molecule-sec Half-Life 0820 Days 12-hr day.
The structure function relation of Glycosaminoglycans from bovine milk bmGAGs has not been studied in detail. In the present study bmGAGs was isolated and structurally characterized. Chondroitin sulphate was one of the major GAGs present and had 65 of ΔDi-diSB GlcA2 S-GalNAc4S followed by 18 of ΔDi-4SΔ45HexUAα1 3GalNAc.
Further bmGAGs exhibited a marked anti-adipogenic. Benzoic acid solid and 1-Propanol liquid Ester C. Acetic acid liquid and 3-Methyl-1-butanol liquid Group 1 is assigned Esters A and B Group 2 is assigned Esters B and C Circle the 2 esters your group has been assigned.
Group 3 is assigned Esters A and C These assignments will allow some replicate samples in case there are experimental errors. Glacial acetic acid and. Draw the structure for each compound.
The ending -ol indicates an alcohol the OH functional group and the hex- stem tells us that there are six carbon atoms in the LCC. We start by drawing a chain of six carbon atoms. The 2 indicates that the OH group is attached to the second carbon atom.
Finally we add enough hydrogen. Ethanol auch Äthanol Trivialname Alkohol ist ein aliphatischer einwertiger Alkohol mit der Summenformel C 2 H 6 O. Die reine Substanz ist eine bei Raumtemperatur farblose leicht entzündliche Flüssigkeit mit einem brennenden Geschmack und einem charakteristischen würzigen süßlichen Geruch.
Bekannt ist Ethanol als Bestandteil von Genussmitteln und alkoholischen Getränken wie Wein. Crystals were collected soaked briefly in 100 mM MES pH 65 10 PEG 5000 MME 12 1-propanol and 20 glycerol and were subsequently flash-frozen in liquid nitrogen. Diffraction data were.
Formate t-butanol and ethylene glycol were also produced at efficiencies. The yields of 1-propanol were higher for single-crystal-terrace electrodes and for thin films with low edge-site densities than for thin films with high densities of edge sites suggesting that the active sites for reduction of CO 2 to 1-propanol are terraces rather than edges. This structure is also available as a 2d Mol file or as a computed 3d SD file The 3d structure may be viewed using Java or Javascript.
Products Building Blocks Explorer Technical Documents Site Content Papers Genes. Ships Today 39 Product Category. Antibodies 211 primary antibodies 211 bioactive small molecules 23 lipids 9 kits 3 Brand.
Sigma-Aldrich 249 Supelco 2 Biological Source. Solvent Other Names Structure. Class Acetic acid Ethanoic acid CH 3 COOH Calss 3 Acetone 2-Propanone CH 3 COCH 3.
Propy al clohol CH. Visit our Solvent Resource Center for more information about our top solvents including synonyms CAS numbers and physical and chemical properties. When you need to buy lab chemicals shop our wide selection of solvents for your chromatography LC LCMS GC protein purification life sciences organic synthesis and general laboratory workflows and applications.
Products Building Blocks Explorer Technical Documents Site Content Papers Genes. Ships Today 16 Product Category. Inorganic acids 16 acids 2 Brand.
Sigma-Aldrich 16 Supelco 9 Boiling Point C Feature. Analytical 4 ACS reagent 2. 1-propanol n-propyl alcohol CH3CH2CH2OH 2-propanol isopropyl alcohol Rubbing alcohol is 70 isopropyl alcohol and 30 water CH3CHCH3 OH.
Chapter 3 Alcohols Phenols and Ethers 4 7 Nomenclature of Alcohols and Phenols Step 1. Name the longest chain to which the hydroxyl OH group is attached. The name for this chain is obtained by dropping the final -e from the name of the.
7Hua Yan Chunxiang Lu Deqi Jing Xianglin Hou Chemical degradation of TGDDMDDS epoxy resin in supercritical 1-propanol. Promotion effect of hydrogenation on thermolysis Polymer Degradation and Stability 2013 9812 25712582 8Hao Junjie Lu Chunxiang Zhou. However MEAs with 1-propanolwater suffered more local site degradation due to inferior ionomer and Pt distribution 71.
Also crucial is the ink-dispersion methodology such as ball milling high. The crystal structure may have changed. A less pure sample may have been used the first time.
The sample may have decomposed slightly. The sample may have decomposed slightly. Melting points can provide the following information about a sample.
Thin layer chromatography can be used to help determine select all correct answers the melting point of a compound.