The equilibrium may be stable or metastable. 89138 gmol 1.

Normally stable even under fire conditions.
1 propanol solubility. 1-Propanol is a primary alcohol with the formula CH 3 CH 2 CH 2 OH and sometimes represented as PrOH or n-PrOHIt is a colorless liquid and an isomer of 2-propanolIt is formed naturally in small amounts during many fermentation processes and used as a solvent in the pharmaceutical industry mainly for resins and cellulose esters and sometimes as a disinfecting agent. Propan-1-ol is the parent member of the class of propan-1-ols that is propane in which a hydrogen of one of the methyl groups is replaced by a hydroxy group. It has a role as a protic solvent and a metabolite.
It is a short-chain primary fatty alcohol and a member of propan-1-ols. 97 C Food and Agriculture Organization of the United Nations 1-Propanol 97 C OU Chemical Safety Data No longer updated More details 97 C Alfa Aesar A19902 43848 22933 22932 41465 41842. 1-Propanol Revision Date 18-Jan-2018 effects vapor concentrations may cause symptoms like headache dizziness tiredness nausea and vomiting Notes to Physician Treat symptomatically 5.
Fire-fighting measures Suitable Extinguishing MediaCO 2 dry chemical dry sand alcohol-resistant foamCool closed containers exposed to fire with water spray. Solubility acidity or basicity stability reactivity towards a particular species such as H etc. Predicting the solubility of an organic molecule is a very useful skill.
For example most reactions are carried out in solution where a solvent needs to dissolve the reactents to allow the molecules to be in the same phase so that they can collide with each other and then react the solvent. Phenylpropanol is an organic molecular entity. 1 Structures Expand this section.
2 Names and Identifiers Expand this section. 3 Chemical and Physical Properties Expand this section. 4 Spectral Information Expand.
The solubility of a solute 1 solid liquid or gas is the analytical composition of a saturated solution expressed in terms of the proportion of the designated solute in a designated solvent 7. Saturated implies equilibrium with respect to the processes of dissolution and demixing. The equilibrium may be stable or metastable.
C 4 H 11 N O. 89138 gmol 1. 3031 C 8688 F.
303304 K Miscible Solubility in alcohols. Soluble Hazards NFPA 704 fire diamond. 22-dimethyl-1-propanol 113 081 35 cyclopentanol 141 095 1-hexanol 156 082 06 cyclohexanol 162 096 36 1-heptanol 176 082.
Group because it forms hydrogen bonds with water and enhances the solubility of an alcohol in water. Methanol ethanol n-propyl alcohol isopropyl alcohol and t-butyl alcohol are all miscible with water. Alcohols with higher molecular weights tend to be.
Illustrations of solubility concepts. Metabolic intermediates lipid bilayer membranes soaps and detergents. Because water is the biological solvent most biological organic molecules in order to maintain water-solubility contain one or more charged functional groups.
These are most often phosphate ammonium or carboxylate all of which are charged when dissolved in an aqueous solution. The current work involves a comprehensive experimental cum theoretical investigation on the equilibrium solubility of CO 2 pertaining to aqueous N-3-aminopropyl-13-propanediamine and its blends with N-methyldiethanolamine and 2-amino-2-methyl-1-propanol. The analysis was conducted within the operating temperature and CO 2 partial pressure range of 3032-3232 K and 2-200 kPa.
1-Propanol 40 16 44 58 2-Propanol 41 16 43 59 1-Butanol 43 15 42 2-Butanol 44 16 40 Benzyl alcohol 48 16 36 60 Cyclohexanol 50 12 38 61 n-amyl alcohol 46 13 41 62 Diacetone alcohol 45 24 31 2-Ethyl-1-hexanol 50 9 41 Polyhydric Alcohols. 63 Ethylene glycol 30 18 52 64 Glycerol 25. Solubility is the ability of a solid to dissolve in a liquid.
Miscibility refer only to liquids. It is the ability of a liquid to mix and form a homogeneous solution soluble in any proportion. Immiscibility refer to those liquids that cannot mix to form a homogeneous solution soluble in all proportions.
Immiscible solvents are incapable of mixing with another solvent and will separate out into. Click to download pdf versionThis solubility list is based on the Hansen Solubility Parameters and should be used as a guide in methods development. The nuances of particle solubility in a given solvent should ultimately be investigated by the experimenter during assay optimization and this guide is not a substitute for bench top evaluationAdapted from.
Properties of Organic Solvents. The values in the table below except as noted have been extracted from online and hardbound compilations. Values for relative polarity eluant strength threshold limits and vapor pressure have been extracted from.
Christian Reichardt Solvents and Solvent Effects in Organic Chemistry Wiley-VCH Publishers 3rd ed 2003. Parameters are given for the hydrogen sulfide solubility in aqueous solutions of monoethanolamine diethanolamine N-methyldiethanolamine 2-amino-2-methyl-1-propanol piperazine diglycolamine di-isopropylamine 1-amino-2-propanol and their selected blends. The model shall be useful for the design of alkanolamine based natural gas sweetening systems operating at high.
C-17 Solubility of Sulfur Compounds in Water as a Function of Boiling Point for Mercaptans and Aromatics C-18 Solubility of Naphthenes in Water C-19 Solubility of Nitrogen Compounds in Water C-20 Henrys Law Constant for Nitrogen Compounds in Water C-21 Coefficient of Thermal Expansion of Liquids C-22 Adsorption Capacity of Activated Carbon FURTHER READING 1. 2-Methyl-1-propanol Isobutyl alcohol 3D. 2-Methyl-1-propanol or isobutanol or isobutyl alcohol is a three-carbon chain with the OH group on and end carbon and a methyl group on the middle carbon.
It is used as a solvent in paints and inks and in the manufacture of some coatings and varnishes. Diamond Hazard Value Description. 3 1 0 Health 1.
Can cause significant irritation. Can be ignited under almost all ambient temperature conditions. Normally stable even under fire conditions.
Special NFPA 2010 General Description. A clear colorless liquid with a sharp musty odor like rubbing alcohol. 1-propanol n-propyl alcohol CH3CH2CH2OH 2-propanol isopropyl alcohol Rubbing alcohol is 70 isopropyl alcohol and 30 water CH3CHCH3 OH.
Chapter 3 Alcohols Phenols and Ethers 4 7 Nomenclature of Alcohols and Phenols Step 1. Name the longest chain to which the hydroxyl OH group is attached. The name for this chain is obtained by dropping the final -e from the name of the.
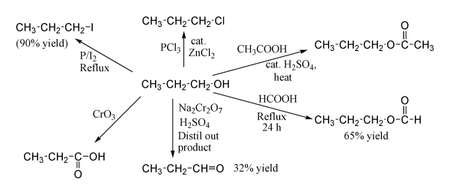
Reaction of propene with diborane followed alkaline hydrolysis in the presence of hydrogen peroxide gives A 1-propanol B 2-propanol C. Xiii 2-Chloropropane to 1-propanol xiv Isopropyl alcohol to iodoform xv Chlorobenzene to p-nitrophenol xvi 2-Bromopropane to 1-bromopropane xvii Chloroethane to butane xviii Benzene to diphenyl xix tert-Butyl bromide to isobutyl bromide xx Aniline to phenylisocyanide. Q-Henrys law constant for CO 2 in water is 167 x 10 8 Pa at 298 K.
Calculate the quantity of CO 2 in 500 mL. An analogous table which also includes Hansen Solubility Parameters and surface tension components can be seen here. If you are concerned with formulation problems which involve surface wetting solubility and viscosity control you may wish to consult both tables.
Each one can be sorted by column just click on the column header as desired to re-order this printable table. 1-propanol acetic acid ethanoic acid propyl acetate propyl ethanoate water. Butan-1-ol 1-butanol acetic acid ethanoic acid butyl acetate butyl ethanoate water.
When the reaction reaches equilibrium there is still a large amount of reactants left in the mixture resulting in a poor yield of the ester. The yield of ester can be improved by increasing the.