
PRoBLem 2 SoL veD Using the pK a values of the conjugate acids of the leaving groups the p a of HBr is K-9 and the pK a of H 2O is 157 explain the difference in reactivity between CH 3Br and. Sugars often lack charged groups but as we discussed in our thought experiment with.
Names originally in other than.
1 butanol solubility in water. 1-Butanol is also formed during deep frying of corn oil cottonseed oil trilinolein and triolein. Butan-1-ol is one of the fusel alcohols from the German for bad liquor which include alcohols that have more than two carbon atoms and have significant solubility in water. 1-Butanol Spectranalyzed Revision Date 08-May-2019 General Advice If symptoms persist call a physician.
Eye Contact Rinse immediately with plenty of water also under the eyelids for at least 15 minutes. Skin Contact Wash off immediately with plenty of water for at least 15 minutes. If skin irritation persists call a physician.
Butan-1-ol is a primary alcohol that is butane in which a hydrogen of one of the methyl groups is substituted by a hydroxy group. It it produced in small amounts in humans by the gut microbes. It has a role as a protic solvent a human metabolite and a mouse metabolite.
2-Methyl-1-butanol IUPAC name also called active amyl alcohol is an organic compound with the formula CH3CH2CHCH3CH2OH. It is one of several isomers of amyl alcoholA colorless liquid it occurs naturally in trace amounts and has attracted some attention as a potential biofuel exploiting its hydrophobic gasoline-like and branched structureIt is chiral. At 20 its solubility in water is 77 by weight while the water solubility in 1-butanol was 201 by weight.
It is miscible with ethanol ether and other kinds of organic solvents. It can be used as the solvents of a variety of paints and the raw material for producing the plasticizers dibutyl phthalate. It can also be used for the manufacture of butyl acrylate butyl acetate and.
Based upon a water solubility of 30000 mgl at 25 C1 the BCF for 2-methyl-1-butanol can be estimated to be 18 from a regression-derived equation2SRC. Based upon a measured log Kow of 1293 the BCF for 2-methyl-1-butanol can be estimated to be 56 from a regression-derived equation2SRC. These BCF values suggest that 2-methyl-1-butanol will not bioconcentrate.
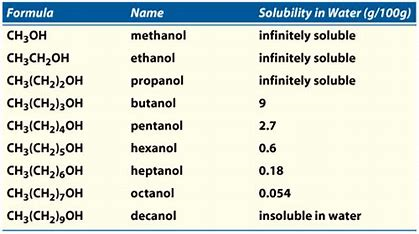
Water is the single most abundant and important liquid on this planet. The miscibility of other liquids in water and the solubility of solids in water must be considered when isolating and purifying compounds. To this end the following table lists the water miscibility or solubility of an assortment of low molecular weight organic compounds.
The influence of the. 1-butanol 73 1-pentanol 22 1-hexanol 59 1-octanol 03 1-nonanol 013 1-decanol 0037 1-dodecanol 0004 The polar hydroxyl group -OH can interact favourably with the structurally similar water molecules via hydrogen bonding. Therefore short chain alcohols are water soluble but as the organic part gets larger ie.
Has more C atoms a longer chain in this series then this interaction is. Because water is the biological solvent most biological organic molecules in order to maintain water-solubility contain one or more charged functional groups. These are most often phosphate ammonium or carboxylate all of which are charged when dissolved in an aqueous solution buffered to pH 7.
Sugars often lack charged groups but as we discussed in our thought experiment with. C 6 H 12 O. Deuterated 2H compounds follow immediately the corresponding H compounds.
References are abbreviated in the forms given by Chemical Abstracts Service Source Index CASSI. Names originally in other than. Hildebrand solubility parameter EXAMPLE.
Nitroethane and 1-butanol have the same Hildebrand solubility parameter 23 MPa 12. Neither will dissolve epoxy resin alone but a blend of the two will Hildebrand recognized this and tried to address it by further classifying compounds according to hydrogen bonding ability weak moderate strong. Safamirzaei et al.
Modeled H 2 solubility in several alcohols named ethanol methanol 1-butanol and 1-propanol applying simple correlations EOSs and artificial neural networks ANNs. The results of this study showed that ANNs have better performance and accuracy than other methods in modeling H 2 in alcohols 26. 1-butanol n-butyl alcohol CH 3 CH 2 3 OH 90 2-butanol sec-butyl alcohol CH 3CHOHCH 2 CH 3.
Group because it forms hydrogen bonds with water and enhances the solubility of an alcohol in water. Methanol ethanol n-propyl alcohol isopropyl alcohol and t-butyl alcohol are all miscible with water. Alcohols with higher molecular weights tend to be less.
Properties of Organic Solvents. The values in the table below except as noted have been extracted from online and hardbound compilations. Values for relative polarity eluant strength threshold limits and vapor pressure have been extracted from.
Christian Reichardt Solvents and Solvent Effects in Organic Chemistry Wiley-VCH Publishers 3rd ed 2003. C-16 Solubility of Selected Gases in Water as a Function of Temperature C-17 Solubility of Sulfur Compounds in Water as a Function of Boiling Point for Mercaptans and Aromatics C-18 Solubility of Naphthenes in Water C-19 Solubility of Nitrogen Compounds in Water C-20 Henrys Law Constant for Nitrogen Compounds in Water C-21 Coefficient of Thermal Expansion of Liquids C-22 Adsorption. 109 C 1013 hPa Color.
1000 gcm3 20 C Flashpoint. Water Standards for KF Titration. Solubility in Water.
Looking at the structure of 1-butanol can you rationalize your observations using IMFs on the miscibility with water and hexanes. Take a closer look at the solubility of 1-butanol and 2. The water should NOT be boiling.
If your water ba th becomes too hot remove the test tubes until the water is in the appropriate temperature range. While your esters are heating answer the questions below the data table for Part A. After heating remove the test tubes from the water bath.
Pour each ester into a separate beaker containing about 2 mL of distilled water this helps with. Solubilities are in water and are reported as grams solvent100 grams water. The water solubility of THF is complex.
T 20 C unless specified otherwise. You can find more detailed information Health Safety Physical Regulatory Environmental on. RhB is a fluorescent dye with good solubility in water and high stability to light chemical formula.
C 28 H 31 ClN 2 O 3. Rh6G is a fluorescent dye used in luminescence microscopy. Unlike RhB it is less sensitive to temperature changes.
The emission of Rh6G is red-shifted as compared with fluorescein. Like fluorescein Rh6G has a high quantum yield C 28 H 31 N 2 O 3 Cl. At the same time.
Chemical Manufacturing and Synthesis. Typical Properties Form Liquid. PKa 25C 97.
Boiling point of active material C 165. Flash point Tag closed cup C 81. Solubility in water Miscible Values shown are typical properties and are not to be considered product specifications.
Test methods available upon request. Test the solubility of each of the listed substance with water by adding 10 drops of the substance to be tested to a test tube. And the kinetics to form H 2 CO 3 are relatively slow on the time scale of seconds.
Give an example of each. For example aqueous acid often abbreviated H 3 O will open an epoxide under MUCH milder conditions than an ordinary. Physical Properties of Amines.
Water Solubility 1 2 and 3 amines can all form hydrogen bonds with water. Low-molecular weight amines are generally water-soluble. CH3 N H H H O H O H H O HH CH3 N CH3 H H O H O H H CH3 N CH3 CH3 H O H 20 Physical Properties of Amines.
Odor Low molecular-weight amines tend to have sharp penetrating odors similar to ammonia Higher. Weakly basic water is the leaving group that is expelled. Because the test relies on the complete solubility of the alcohol in Lucas reagent it is limited to alcohols with fewer than six carbons.
PRoBLem 2 SoL veD Using the pK a values of the conjugate acids of the leaving groups the p a of HBr is K-9 and the pK a of H 2O is 157 explain the difference in reactivity between CH 3Br and.