Concentration information is not available for. Se ha informado de reacciones explosivas durante la nitración de este producto químico con ácido nítrico y sulfúrico cuando las condiciones no son controladas cuidadosamente.
12 complete 036 090.
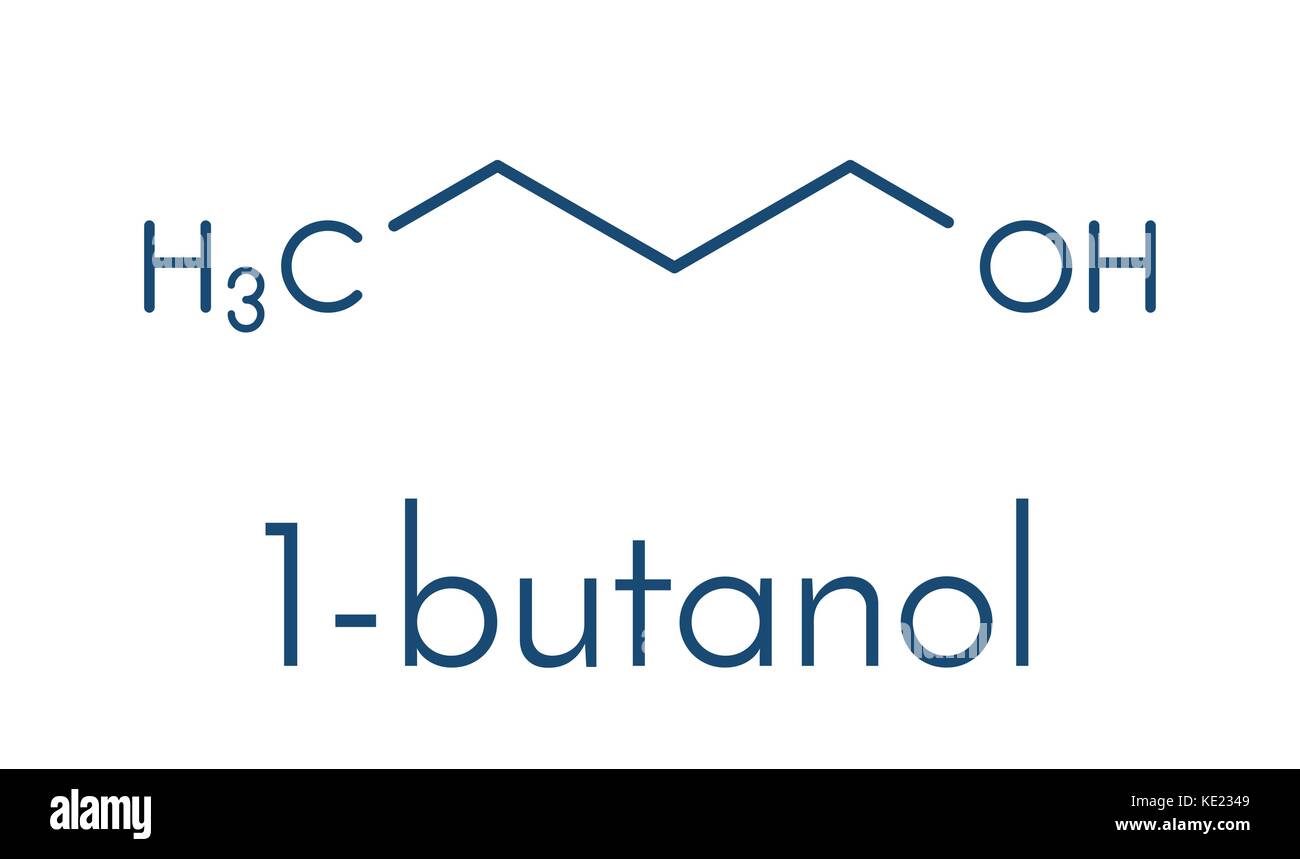
1 butanol formula. Butan-1-ol also known as n-butanol is a primary alcohol with the chemical formula C 4 H 9 OH and a linear structure. Isomers of butan-1-ol are isobutanol butan-2-ol and tert-butanol. The unmodified term butanol usually refers to the straight chain isomer.
1-Butanol occurs naturally as a minor product of the ethanol fermentation of sugars and other saccharides and is present in many foods and. Temperature K A B C Reference Comment. Kemme and Kreps 1969.
Hessel and Geiseler 1965. 2-Methyl-1-butanol IUPAC name also called active amyl alcohol is an organic compound with the formula CH3CH2CHCH3CH2OH. It is one of several isomers of amyl alcoholA colorless liquid it occurs naturally in trace amounts and has attracted some attention as a potential biofuel exploiting its hydrophobic gasoline-like and branched structureIt is chiral.
Except where noted spectra from this collection were measured on dispersive instruments often in carefully selected solvents and hence may differ in detail from measurements on FTIR instruments or in other chemical environments. More information on the manner in which spectra in this collection were collected can be found here. Concentration information is not available for.
Butan-1-ol is a primary alcohol that is butane in which a hydrogen of one of the methyl groups is substituted by a hydroxy group. It it produced in small amounts in humans by the gut microbes. It has a role as a protic solvent a human metabolite and a mouse metabolite.
1-Butanol Spectranalyzed Revision Date 08-May-2019 Autoignition Temperature 340 C 644 F Decomposition Temperature No information available Viscosity 295 mPas 20 C Molecular Formula C4 H10 O Molecular Weight 7412 Refractive index 1390 - 1400 10. Stability and reactivity Reactive Hazard None known based on information available Stability Stable under normal conditions. Visit ChemicalBook To find more 1-Butanol71-36-3 information like chemical propertiesStructuremelting pointboiling pointdensitymolecular formulamolecular weight physical propertiestoxicity informationcustoms codes.
You can also browse global suppliersvendorpricesPricemanufacturers of 1-Butanol71-36-3. Although biodegradation data specific to 2-methyl-1-butanol are unavailable analogy to similar aliphatic alcohols suggests that 2-methyl-1-butanol will be readily biodegradable. The volatilization half-lives of 2-methyl-1-butanol from a model environmental river 1 meter deep and model pond have been estimated to be 61 hr and 28 days respectively12SRC.
El butan-1-ol es un alcohol de fórmula H 3 C-CH 2 3-OHLos isómeros de este compuesto son el butan-2-ol el metilpropan-1-ol y el metilpropan-2-olSe cataloga como alcohol primario porque el grupo hidroxilo está unido a un carbono primario. Y como un alcohol de fusel al tener más de dos carbonos. El butan-1-ol se encuentra naturalmente como subproducto de la fermentación de azúcares.
As we can see above ethyl alcohol and dimethyl ether have different structural formula. But their molecular formula is same which is C 2 H 6 O. These types of compounds have not only different structural formulas but shows distinct properties as well.
Thus we can say a molecular formula can represent more than one compound. This phenomenon is known as isomerism. If we mentally insert an oxygen atom into a C-H bond we will have an alcohol either 1-butanol or 2-butanol.
Both compounds have zero degrees of unsaturation no multiple bonds or rings. If the oxygen atom were inserted in a C-C bond of n-butane ethers would be formed both of which diethyl etherand methyl n-propyl ether have zero degrees of unsaturation. Veja que a fórmula mínima CH 2 O é a mesma para todas as substâncias.
Isso porque ela expressa que em todos os casos os átomos de carbono hidrogênio e oxigênio estão presentes nas fórmulas moleculares em uma relação de 121. Além disso o único que apresenta a fórmula molecular igual à fórmula empírica é o formaldeído. Alcohol - alcohol - Physical properties of alcohols.
Most of the common alcohols are colourless liquids at room temperature. Methyl alcohol ethyl alcohol and isopropyl alcohol are free-flowing liquids with fruity odours. The higher alcoholsthose containing 4 to 10 carbon atomsare somewhat viscous or oily and they have heavier fruity odours.
We are a leading supplier to the global Life Science industry with solutions and services for research biotechnology development and production and pharmaceutical drug therapy development and production. Write a condensed structural formula such as CH 3 CH 3 and describe the molecular geometry at each carbon atom. Write a condensed structural formula such as CH 3 CH 3 and describe the molecular geometry at each carbon atom.
Add to Cart star Add to Favorites. Details Availability –Epigallocatechin-3-O-gallate 20 mg Net Weight. Add to Cart star Add to Favorites.
Details Availability E-Thiothixene 100 mg Net Weight. Formula Name taz C x1 Water H 2O CHCl3. 1-Butanol C4H10O C5H5N Pyridine 1186 0704 C6H5Cl Chlorobenzene 1153 0659 C6H10 Cyclohexene 82 0055 C7H8 Toluene 1055 0324 C7H16 Heptane 939 0229 C8H10 12-Dimethylbenzene 1168 0811 C8H18O Dibutyl ether 1177 0892-4-AZEOTROPIC DATA FOR BINARY MIXTURES continued Molecular formula Name tazC x1 2-Butanol C4H10O.
Empirical Formula Hill Notation. C 27 H 42 N 7 Na 2 O 20 P 3 S xH 2 O. 95562 anhydrous basis Compare Product No.
Ac-CoA Synthase Inhibitor - CAS 508186-14-9 - Calbiochem. Ac-CoA Synthase Inhibitor - CAS 508186-14-9 - Calbiochem. En este ejemplo primero se nombran el radical metil seguido de la posición del grupo hidroxil junto al nombre del hidrocarburo terminado en ol por lo tanto su nombre es 2-metil-1-butanol.
Similar al caso anterior presenta el radical bromo en la posición 3 y el grupo hidroxil en la 2 por lo tanto el nombre es 3-bromo-2-butanol. C 1-Butanol y etoxietano. Isómeros de función.
La fórmula molecular de estos compuestos es. Sin embargo el primero es un alcohol mientras que el segundo es un éter por lo que son isómeros de función al tener distinto grupo funcional. Solvent Synonyms Mol Wt BP C Linear Formula H-Signal Multi CDCl 3 D 2 O CD 3 OD CD 3 2 SO CD 3 2 CO CD 3 CN C 6 D 6 Acetic Acid Ethanoic acid 6005 118 CH 3COOH CH 3 s 213 208 199 195 196 196 155 Formic Acid Methanoic Acid 4602 101 HCOOH H s 802 822 808 818 811 803 1-Butanol n-Butanol 1-Hydroxybutane n-Butyl alcohol.
1-butanol 1055 72 2-pentanol 107 72 3-pentanol 106 65 PROPIEDADES QUIMICAS. Monóxido y dióxido de carbono. Se ha informado de reacciones explosivas durante la nitración de este producto químico con ácido nítrico y sulfúrico cuando las condiciones no son controladas cuidadosamente.
Reaccion a de la misma. Hints for logging in with Active Directory. 1-butanol tert-butanol cyclohexanol phenol.
90 complete 36 87. 12 complete 036 090. Ethyl ether THF furan anisole.
60 complete 10 10. 009 Compounds Having Two Oxygens. 13-propanediol 2-butoxyethanol butanoic acid benzoic acid.
Complete complete complete complete. Complete complete complete complete. Cloroformo etanol 1-butanol hexano consultar el punto de ebullición y la fórmula química de estos líquidos Aceite mineral Vaso de precipitados tubo de ensayo Soporte universal Termómetro Mechero Capilares traerlos 64 Procedimiento.
A un tubo de ensayo pequeño se añaden 2 mL del líquido problema se introduce un capilar sellado por uno de.